2022 was a relatively slow year when it comes to approvals of new drugs. The US Food and Drug Administration (FDA) approved 37 new molecular entities, compared to 50 in 2021, 53 in 2020, and 48 in 2019. Of the 37 new drugs approved in 2022, 17 were small molecules, accounting for 46%. This is also a decline from 2021 and 2020, when small molecules made up 62 and 66% of the new drugs, respectively.
Quality vs Quantity
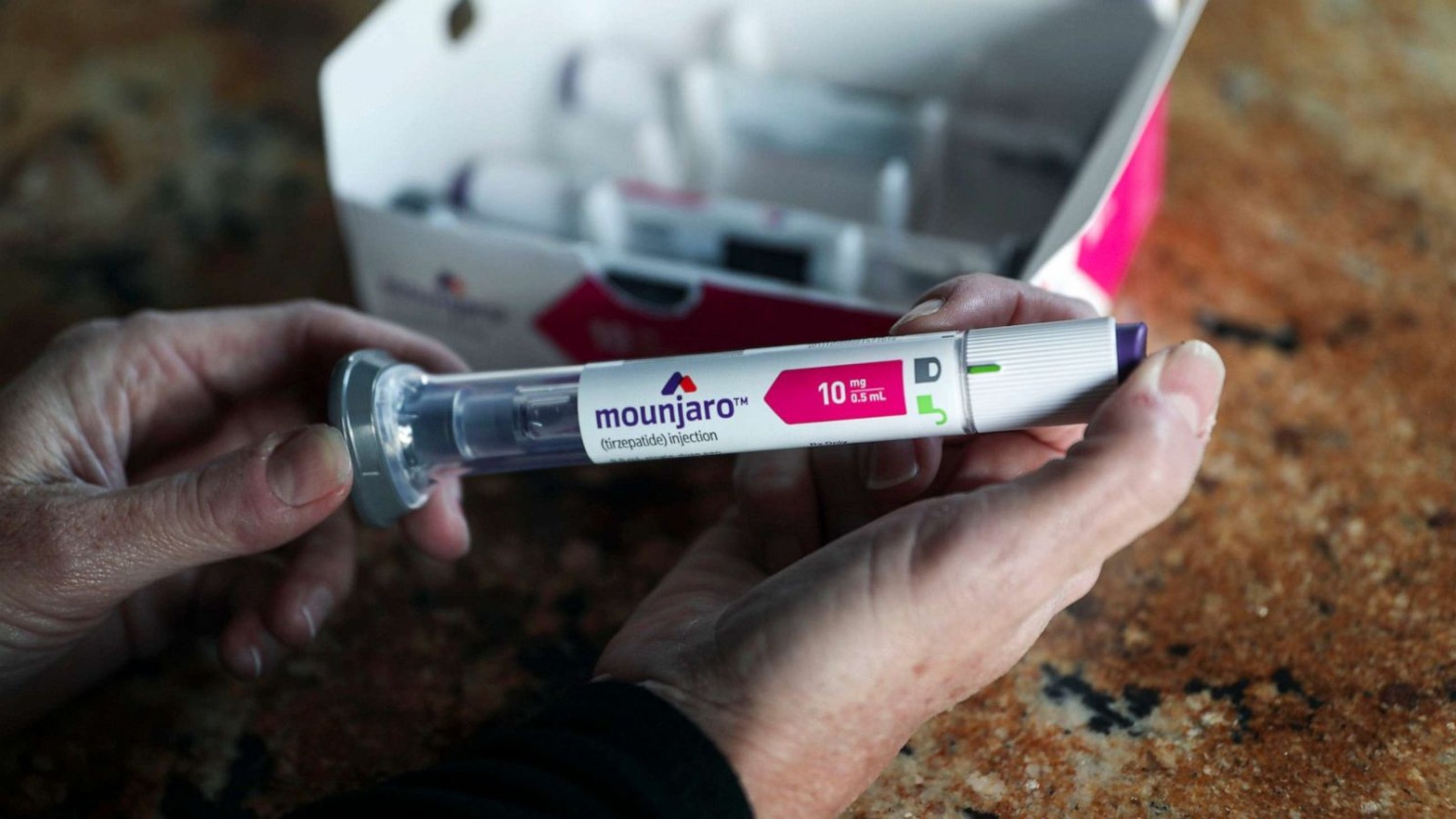
While the number of approvals where lower than yesteryear, the level of innovation has somewhat improved. Take Eli Lilly and Company’s Mounjaro (tirzepatide) for example. It is a weekly injectable for type-2 diabetes, and mimics two gut hormones involved in blood sugar regulation—glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The FDA first approved exenatide, a synthetic version of GLP-1, back in 2005, but Mounjaro is the first to feature a GIP mimic.
Bristol Myers Squibb’s Sotyktu (deucravacitinib), a psoriasis treatment approved in 2022, is the first drug to contain deuterium, a heavier form of hydrogen. Molecule enthusiasts will also appreciate the complex structure of Gilead Sciences’ HIV treatment Sunlenca (lenacapavir), a first-in-class, twice-yearly treatment option for people living with multi-drug resistant HIV.
Recently Approved Drugs of Significance
Some of the more significant FDA approvals as recent as May 2023 were:
- Paxlovid (nirmatrelvir tablets and ritonavir tablets) is a combination antiviral medication that was approved for the treatment of mild to moderate COVID-19 in adults at high risk of progression to severe COVID-19. Paxlovid is the first oral antiviral medication to be approved for the treatment of COVID-19 in the United States.
- Xacdura (sulbactam, durlobactam) is a combination antibiotic that the FDA approved in June 2023 for the treatment of complicated urinary tract infections (UTIs) caused by certain bacteria. Xacdura is the first combination antibiotic to be approved for the treatment of complicated UTIs in the United States.
- Arexvy (respiratory syncytial virus vaccine, adjuvanted) is a respiratory syncytial virus (RSV) vaccine that was approved for the prevention of RSV infection in adults aged 65 years and older. RSV is a common respiratory virus that can cause serious illness in infants, young children, and adults aged 65 years and older. Arexvy is the first RSV vaccine to be approved for adults in the United States.
Paxlovid has the potential to be an important tool in the fight against COVID-19. It is an oral antiviral medication that is taken for five days, and it has been shown to be effective in preventing serious illness and death due to COVID-19. Paxlovid is especially effective in people who are at high risk of severe illness, such as older adults and people with underlying medical conditions.
However, it is important to note that Paxlovid is not exactly a ‘wonder’ cure for COVID-19. While it can help to prevent serious illness, it will not prevent people from getting infected or from spreading the virus to others. It is also not readily available to everyone, prescription is usually limited to those who are at high risk of severe illness and, it also depends on insurance coverage.

The more significant FDA drug approvals in recent years include:
- Aduhelm (aducanumab) is a monoclonal antibody that was approved in June 2021 for the treatment of Alzheimer’s disease. It is the first drug to be approved for Alzheimer’s disease in almost 20 years.
- Evusheld (tixagevimab and cilgavimab) is a combination of monoclonal antibodies that was approved in December 2021 for the prevention of COVID-19. It is the first drug to be approved for the prevention of COVID-19.
- Vyvgart (teplizumab) is a monoclonal antibody that was approved in March 2022 for the treatment of type-1 diabetes. It is the first drug to be approved for the treatment of type 1 diabetes in over 20 years.
- Zeposia (ozanimod) is an oral medication that was approved in March 2022 for the treatment of relapsing-remitting multiple sclerosis. It is the first oral medication to be approved for the treatment of relapsing-remitting multiple sclerosis.
- Krystexxa (pegcetacoplan) is an enzyme replacement therapy approved in April 2022 for the treatment of Fabry disease. It is the first enzyme replacement therapy to be approved for the treatment of Fabry disease (a rare genetic disorder that causes the buildup of fatty substances in the body’s cells).
- Breo Elipta (glycopyrronium/formoterol fumarate) inhalation powder is a combination of two long-acting bronchodilators that was approved in April 2022 for the long-term maintenance treatment of asthma in adults and children 6 years of age and older.
- Oral semaglutide is a once-weekly injectable GLP-1 receptor agonist that was approved in June 2022 for the treatment of adults with obesity or overweight who have obesity-related co-morbidities, such as type 2 diabetes, high blood pressure, or high cholesterol.
- Upadacitinib is a once-daily oral Janus kinase inhibitor that was approved in June 2022 for the treatment of adults with active rheumatoid arthritis who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) inhibitors.
- Acalabrutinib is a once-daily oral Bruton’s tyrosine kinase inhibitor that was approved in July 2022 for the treatment of adults with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy.
- Rucaparib is an oral poly ADP-ribose polymerase (PARP) inhibitor that was approved in July 2022 for the treatment of adult patients with advanced ovarian cancer who have received at least three prior systemic therapies.
These drugs represent a significant advance in the treatment of a variety of diseases. They offer new hope for patients who have previously had few or no treatment options.
Recent Drug Approvals in Europe
The European drug approval market is also seeing a fair share of exciting development, despite the drop in the number of approvals from 54 new active substances in 2021 to 41 in 2022. Below are significant drugs approved by the EMA (European Medicines Agency) this year and the last:
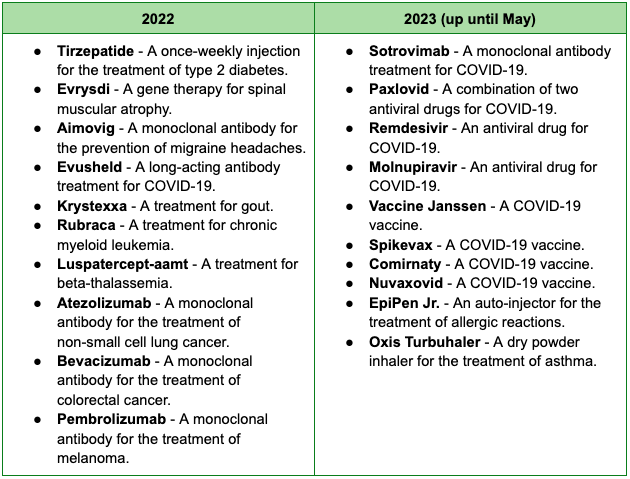
While the FDA is the agency of the United States, the EMA is the agency of the European Union (EU) that is responsible for the evaluation and supervision of medicines before and during they are marketed in the EU. Based in Amsterdam, Netherlands, decisions on new medicines are binding on all EU member states. This means that once the EMA has approved a new medicine, it can be marketed in all EU member states without any further approval from national authorities.
Recently Approved Medical Devices in the United States
According to Emergo by UL, there were a total of 3,229 510(k) clearances in 2022, a significant increase compared to 2,996 clearances in the previous year. Up to 216 Emergency Use Authorizations (EUAs) were granted for medical devices related to COVID-19, with an additional 6 EUAs related to mpox (also known as monkeypox).
Premarket Approvals or PMAs (which are typically meant for Class III, high-risk, and/or novel devices) in 2022 were only 22 when compared to 31 in the previous year.
Some of the more notable 510(k) clearances in recent years are:
- Air Optix contact lens (by Alcon) that can be worn for up to 30 days straight (day and night) without being removed.
- A leadless pacemaker (by Aveir) that can be implanted without surgery.
- Drug-eluting stents (by Medtronic) that can help to prevent heart attacks.
The recent approvals of new drugs and medical devices are a positive sign for the future of healthcare. These new products offer the potential to improve the lives of patients with a wide range of conditions. While 2022 is a slower year for drug approvals compared to 2021, the overall trend remains positive, and it is clear that the life sciences industry is making progress in developing new and innovative treatments for disease.
Sources:
- https://cen.acs.org/content/cen/sections/drugs-approved-in-2022.html
- https://cen.acs.org/pharmaceuticals/37-new-drugs-achieved-FDA/101/i3
- https://www.emergobyul.com/news/us-fda-annual-report-nearly-6000-medical-device-authorizations-2022
- https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
- https://www.drugs.com/newdrugs.html
- https://www.fdanews.com/
- https://www.ema.europa.eu/en/medicine