Addressing a Major Unmet Need in Overcoming Antibiotic Resistance
Antibiotics were long regarded as one of the greatest successes of modern medicine. It is critical in saving millions of lives every day through the treatment of bacterial infections that causes fever, chills, cough, uncontrolled shaking, headaches, and more. Thanks to antibiotics, many medical procedures become possible, from common surgeries (including birth, appendectomy, bypass, etc.), cancer treatments as well as organ transplants.
In this article, we review how a new generation of antibiotics would help address a major unmet need in tackling antibiotic resistance. Before we can talk about Allecra and their new product candidate, let’s take a step back and do a quick recap on antibiotic resistance.
A Recap on Antimicrobial Resistance
Antimicrobial resistance (AMR) happens when microorganisms (including bacteria and viruses) change when they are exposed to antimicrobial drugs (including antibiotics and antivirals). This change happens through a series of antimicrobial drug medications.
For example, when a patient is diagnosed with a bacterial infection and is prescribed antibiotics, the bacteria causing the sickness is killed off. However, some of the pathogens (the bacteria causing the disease) survive and eventually develop a resistance to the said antibiotic.
The drug-resistant genes pass on to their daughter cells as they multiply, eventually rendering the current medication ineffective. Doctors would need to prescribe a stronger dose of antimicrobial drugs, which will cause more microbial species to develop resistance.
Microorganisms that develop AMR are sometimes referred to as “superbugs”.
Antimicrobial Resistance is a Big Deal
Misuse and overuse of antimicrobials (including antibiotics) are the main drivers in the development of antimicrobial-resistant pathogens. WHO has declared that AMR is one of the top 10 global public health threats facing humanity today.
A growing number of infections – such as pneumonia, tuberculosis, gonorrhoea, and salmonellosis – are becoming harder to treat as the antibiotics used to treat them become less effective. AMR leads to longer hospital stays, higher medical costs and increased mortality.
According to the American Centers for Disease Control and Prevention (CDC), more than 2.8 million antibiotic-resistant infections occur in the U.S. each year, and more than 35,000 people die as a result. When Clostridioides difficile (a bacterium that is not typically resistant but can cause deadly diarrhea and is associated with antibiotic use) is added to these, the toll of all the threats in their 2019 AR Threats Report exceeds 3 million infections and 48,000 deaths.
Every country is affected by this growing crisis, and it is estimated that more than 700,000 people globally die each year because of infections caused by resistant bacteria, including 33,000 in Europe alone.
An Overview of Complicated Urinary Tract Infections (cUTIs)
A urinary tract infection (UTI) is an infection in any part of the urinary system — kidneys, ureters, bladder and urethra. Symptoms include:
- Strong, persistent urge to urinate
- Burning sensation when urinating
- Strong-smelling urine
UTI is generally easy to treat with antibiotics but can get complicated (cUTI), and traditional treatment wouldn’t be as effective. β-lactams (pronounced ‘beta lactams’) were once the most widely used antibiotic class because of their known safety and efficacy. Unfortunately, the extensive use of these antibiotics resulted in the emergence of Extended-Spectrum β-lactam resistant pathogens (ESBLs).
Today, ESBLs represent a worldwide problem, with high and growing prevalence in both developed and developing countries. According to the CDC, ESBLs increased >50% over 5 years and were responsible for approximately 197,000 infections in hospitalized patients, around 9,100 deaths and direct healthcare costs of up to USD 1.2 billion in a single year. In Europe, ESBLs have been increasingly reported for decades.
To combat ESBLs, antibiotics that combine β-lactams with a β-lactamase inhibitor, such as piperacillin-tazobactam, or other class of antibiotics such as carbapenems were developed and widely used. Unfortunately, piperacillin/tazobactam is now also suffering from a growing ESBL-mediated resistance and carbapenem use is restricted by hospitals to prevent the growth of additional resistant pathogens.
Allecra’s Cefepime/enmetazobactam
Allecra Therapeutics is a clinical-stage biopharmaceutical company that is developing novel therapies to combat antibiotic resistance. Founded in 2013, their ongoing research seeks to overcome emergent resistance mechanisms. Their lead product candidate is cefepime/enmetazobactam, a combination of enmetazobactam with a 4th generation cephalosporine, cefepime, is intended to provide a therapeutic option addressing the serious threat of ESBLs.
What makes their lead product candidate special? The novel β-lactamase inhibitor enmetazobactam, has potent activity against Class A serine β-lactamases, specifically ESBL. Enmetazobactam is a derivative of tazobactam but from a molecule comparison there are significant differences to expect higher potency:
- It has a strategically placed methyl group that confers stronger interactions with ESBL at the active site.
- It imparts a zwitterionic structure that enhances penetration into the periplasmic space, where β-lactamases are located.
- It also exhibits an extended half-life relative to tazobactam.
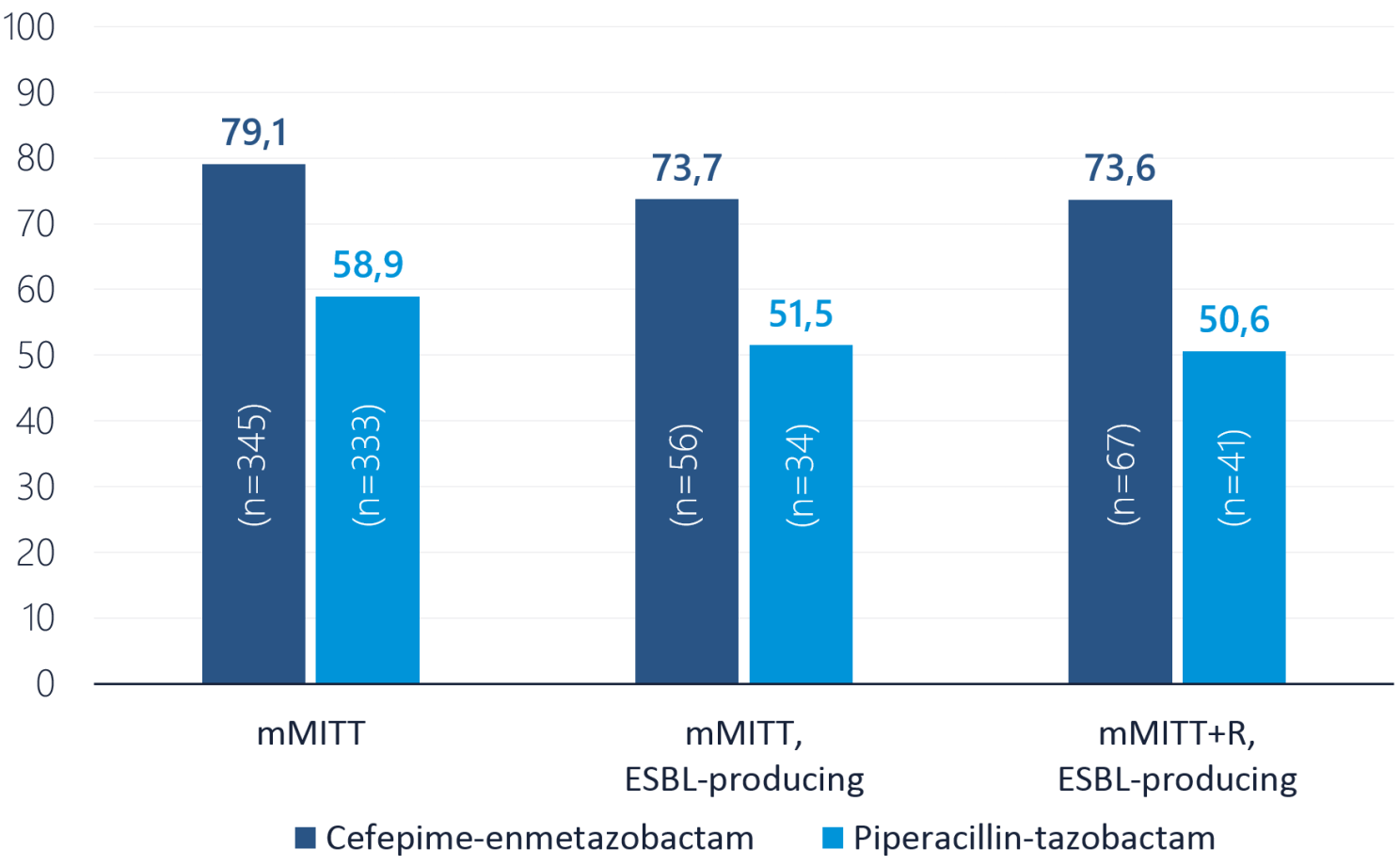
The combination of enmetazobactam with cefepime has demonstrated potent in vitro activity against clinical isolates of Enterobacterales, including ESBL producers. In short, cefepime/enmetazobactam has shown promising results in the standard of care for patients with complicated urinary tract infections (cUTIs) in a recent Phase 3 called ‘ALLIUM’.
The Phase 3 ALLIUM was a multi-center, randomized, controlled, double-blind, global study that enrolled 1,034 patients across 112 sites in 19 countries. In summary, the overall success rate was 79.1% for cefepime/enmetazobactam vs. 58.9% for piperacillin/tazobactam.
Recent Partnerships to Fulfil an Unmet Need
Allecra Therapeutics has recently signed an exclusive license agreement with ADVANZ PHARMA on January 13th, 2022. ADVANZ PHARMA gains the rights to develop and commercialize Allecra’s antibiotic drug candidate cefepime/enmetazobactam within the European Union, the United Kingdom, Switzerland, and Norway.
In addition to ADVANZ PHARMA, Allecra has also signed an exclusive license agreement (back in December 2020) with Shanghai Haini Pharmaceutical to manufacture, develop, and commercialize cefepime/enmetazobactam within Greater China (including Mainland China, Hong Kong, Macau, and Taiwan).
Sources:
- https://www.cdc.gov/drugresistance/biggest-threats.html
- https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
- https://www.allecra.com/
- https://www.allecra.com/more-cefepime
- https://www.healthdirect.gov.au/bacterial-infections
- https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
- https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
- https://www.mayoclinic.org/diseases-conditions/urinary-tract-infection/symptoms-causes/syc-20353447
- https://www.healthline.com/health/urinary-tract-infection-adults/complicated-uti-treatment